 Chinese Healthcare Firm Vivolight's Multimodality OCT Systems Are World's First to Get FDA Approval
Chinese Healthcare Firm Vivolight's Multimodality OCT Systems Are World's First to Get FDA Approval(Yicai) April 16 -- The multimodality optical coherence tomography systems developed by Chinese healthcare solutions provider Vivolight Medical Device and Technology have become the first ever to receive approval from the United States Food and Drug Administration.
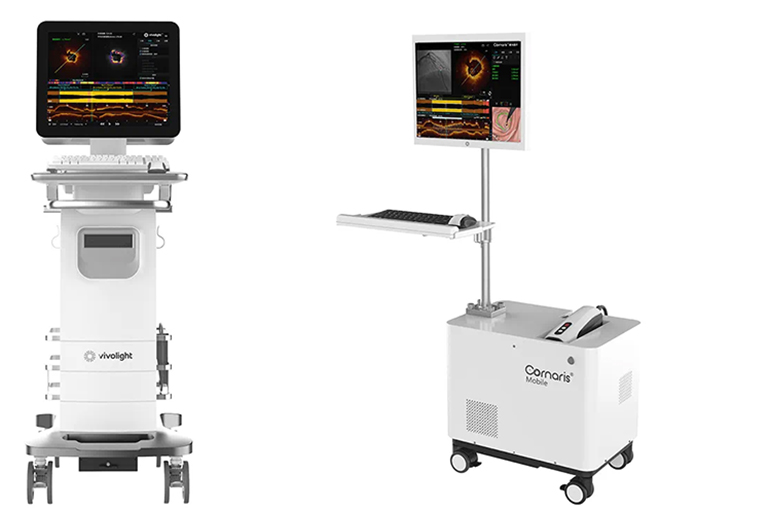
Vivolight's independently developed multimodality OCT systems Cornaris P80 and Cornaris Mobile obtained FDA 510(k) clearance on April 11, the Shenzhen-based company announced yesterday. The FDA also approved Vivolight’s OCT imaging catheter LumenCross F2.
"The FDA 510(k) clearance marks a pivotal achievement in the company's regulatory and commercialization journey, furthering its commitment to delivering world-class imaging tools to interventional cardiologists and patients around the world," Vivolight noted.
"This milestone not only enables the US market entry but also streamlines regulatory pathways across over 20 countries recognizing FDA approvals, significantly advancing the company's global commercialization strategy," it added.
Cornaris P80 and Cornaris Mobile are advanced intravascular imaging devices designed for comprehensive diagnostic capabilities, according to Vivolight's website. They integrate multiple techniques, offering unparalleled and detailed visualization of vascular tissue structures for percutaneous coronary intervention guidance.
Multimodality refers to the three novel technologies integrated into one OCT platform, which are index of plaque attenuation technology for plaque stability assessment, virtual flow ratio technology for functional assessment of coronary stenosis, and intelligent calcium assessment technology for calcium severity, Vivolight's website also shows.
Compared to angiography, OCT reduces procedural uncertainties by providing layered arterial views. Moreover, multimodality OCT devices can significantly lower the operation time and cut the cost of consumables compared with single-technique OCT devices provided by Vivolight’s competitors like Abbott Laboratories and Terumo.
Vivolight has seized a major share of the Chinese OCT device market, ranking second after Abbott. The compound annual growth rate of its OCT device sales exceeded 150 percent in the past three years. In 2023, the company became the first Chinese OCT manufacturer to foray into overseas markets.
The biggest challenge restricting the widespread application of intravascular imaging devices such as OCT systems is their relatively high price, according to clinical practice surveys conducted by the European Association of Percutaneous Cardiovascular Interventions and the Cardiovascular Intervention and Therapeutics Society of Japan.
Vivolight has greatly reduced the production cost of its OCT devices because their components, including catheters, are made by the company or domestically produced, while all their core modules are manufactured by robots, founder and Chief Executive Zhu Rui told Yicai.
"Only inexpensive, high-quality products can have core competitiveness," he noted, adding that Vivolight is expected to effectively deal with the US import tariff barriers in the volatile foreign trade environment.
Editors: Tang Shihua, Futura Costaglione