 First China-Developed Extracorporeal Life Support Machine Gets Marketing Approval
First China-Developed Extracorporeal Life Support Machine Gets Marketing Approval(Yicai Global) Jan. 5 -- China’s National Medical Products Administration today announced the emergency approval of Chinabridge Shenzhen Medical Technology’s registration of an extracorporeal membrane oxygenation machine.
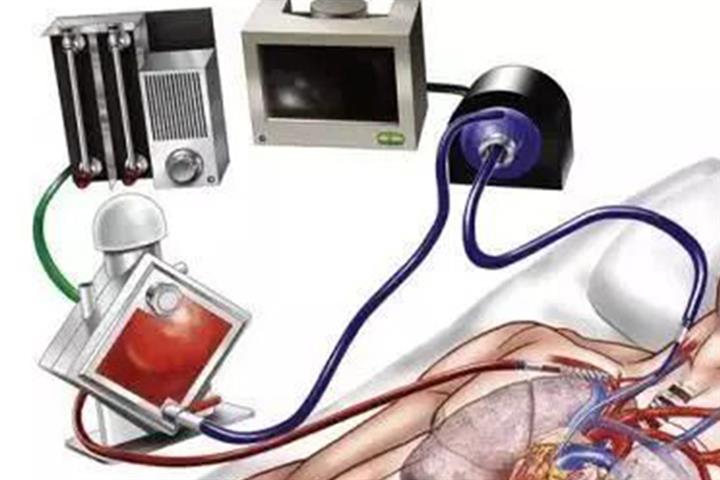
This is the first Chinese ECMO approved for marketing. The ones currently being used by Chinese medical institutions are mainly imported. Medtronic, LivaNova, and Maquet are the main makers of ECMOs worldwide.
According to the regulator, as the first domestic ECMO equipment and consumables package, the devices performance indicators have basically reached the international level for the same type of product.
ECMOs can effectively avoid hypoxemia in patients and buy time for cardiorespiratory recovery. Because they play an important role in the survival of critically ill patients with Covid-19, they have attracted much market attention.
The regulator noted that the ECMO is a device for the critically ill with Covid-19 for whom conventional treatment is ineffective. Domestic products are needed to meet urgent clinical needs and ensure the treatment of severe patients, it added.
Founded in 2018, Chinabridge Shenzhen Medical Technology is one of the few tech firms in China to develop the overall ECMO system and has applied for 77 related intellectual property rights.
Editor: Shi Yi, Peter Thomas