 China's Medical Insurance Will Not Cover Fosun Kite’s Costly CAR-T Cell Therapy
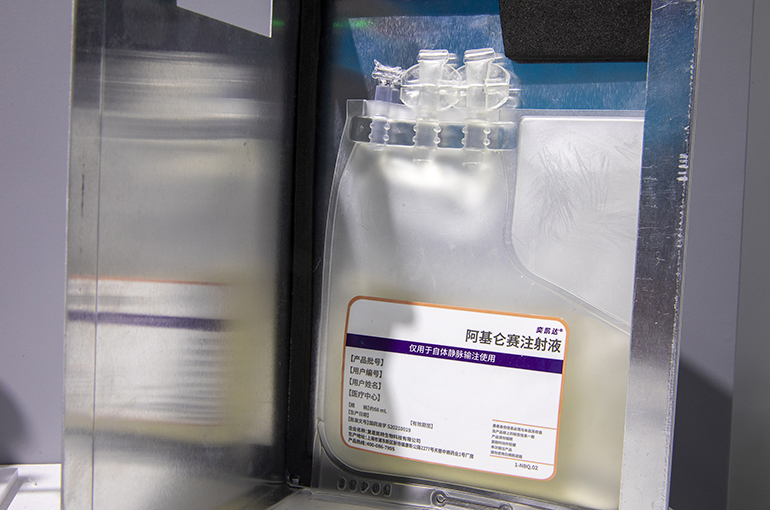
China's Medical Insurance Will Not Cover Fosun Kite’s Costly CAR-T Cell Therapy(Yicai) Nov. 17 -- Fosun Kite Biotechnology’s Yescarta, China's first approved chimeric antigen receptor-T cell therapy, has failed to enter the country's medical insurance program, Yicai learned exclusively.
The CAR-T cell therapy that costs over CNY1 million (USD138,000) is not mentioned in the negotiation list. CAR-T cell therapy is a novel way to use the body's own immune system to help fight cancer.
China has so far approved four CAR-T cell therapies, all designed to treat hematologic cancers that start in the immune system or in blood-forming tissue. Yescarta, developed by the joint venture between American Kite Pharma and Shanghai-based Fosun Pharmaceutical Group, was given the green light in June 2021.
Yescarta and Carteyva, a CAR-T therapy developed by JW Therapeutics, were on the post-review list that the National Healthcare Security Administration disclosed on Sept. 1 but it does not mean they will make it to the negotiation phase.
Shanghai-based JW Therapeutics told Yicai that it is not convenient to disclose more details right now after a rumor claimed that the firm's Carteyva also failed to move to the next step in the process.
An industry insider believed that the two medicines could be unsuccessful this time because they cost more than CNY300,000 (USD41,405) even after price cuts. So far all the drugs in the public medical insurance program are priced lower than that.
CAR-T cell therapy re-engineers a patient's cells so the products cannot be mass-produced like traditional medicines so treatments are generally priced at around CNY1.2 million (USD165,000) in China. Given the high production costs, it is rather difficult to reduce the price for existing CAR-T cell therapies, the insider added.
Editors: Shi Yi, Emmi Laine